ABSTRACT
OBJECTIVES: to describe estimated fetal weight, biparietal diameter, head circumference, abdominal circumference, and femoral length in a sample of pregnant women and to compare them with the international curves.
METHODS: a retrospective cross-sectional study was conducted on ultrasonographic data of singleton pregnancies between 16 and 39 weeks in Bogotá, Colombia, from February 2015 to November 2018. Fetal parameters were evaluated. Descriptive analysis of each biometric parameter was performed, followed by comparison the curves provided by INTERGROWTH-21st and Lagos.
RESULTS: a total of 1133 sonographic reports were analysed. The means ± SDs of biparietal diameter, head circumference, abdominal circumference, femur length, and estimated fetal weight measurements at 16 and 39 weeks were 34.7 ± 1.5 and 92.2 ± 4.4 mm, 122.2 ± 6.6 and 318.0 ± 17.0 mm, 107.2 ± 6.8 and 329.3 ± 34.6 mm, 20.6 ± 2.8 and 73.5 ± 3.3 mm, and 257.8 ± 20.9 and 3,115 ± 663.7g, respectively.The data were presented in graphs. AC and FL were the parameters that showed more statistically significant differences with international curves.
CONCLUSIONS: international reference charts analysed, show differences when fetal growth of this population was compared. The customized or local charts maybe are more useful to reach early detection of alterations of fetal growth in each population.
Keywords:
Fetal weight, Growth charts, Ultrasonography, Prenatal, Reference standards
RESUMO
OBJETIVOS: describir el peso fetal estimado (PFE), el diámetro biparietal (DBP), la circunferencia cefálica (CC), la circunferencia abdominal (CA) y la longitud femoral (LF) en una muestra de gestantes y compararlos con curvas internacionales.
MÉTODOS: se realizó un estudio transversal retrospectivo sobre datos ultrasonográficos de embarazos únicos entre 16 y 39 semanas en Bogotá, Colombia, desde febrero del 2015 hasta noviembre del 2018. Se realizó un análisis descriptivo de cada parámetro biométrico, seguido de una comparación con las curvas proporcionados por INTERGROWTH-21st y Lagos. Resultados: se analizaron un total de 1133 informes ecográficos. Las medias ± DE de las mediciones del DBP, CC, CA, LF y PFE a las 16 y 39 semanas fueron 34,7 ± 1,5 y 92,2 ± 4,4 mm, 122,2 ± 6,6 y 318,0 ± 17,0 mm, 107,2 ± 6,8 y 329,3 ± 34,6 mm, 20,6 ± 2,8 y 73,5 ± 3,3 mm, y 257,8 ± 20,9 y 3115 ± 663,7 g, respectivamente. La CA y LF fueron los parámetros que mostraron más diferencias estadísticamente significativas con las curvas internacionales.
CONCLUSIONES: los cuadros de referencia analizados muestran diferencias al comparar el crecimiento fetal de esta población. Las gráficas personalizadas o locales quizás sean más útiles para detectar tempranamente alteraciones del crecimiento fetal en cada población.
Palavras-chave:
Peso fetal, Gráficos de crecimiento, Ultrasonografía, Prenatal, Estándares de referencia
IntroductionThe evaluation of fetal growth (FG) through ultrasound is crucial in current obstetric practice. The alteration of fetal growth increases the risk of complications during pregnancy, childbirth and the neonatal period.
1,2,3In 2000, Goodfrey, proposed that fetuses with growth alterations often exhibit changes in their physiology and metabolism, potentially predisposing them to chronic adult pathologies like hypertension, diabetes, obesity, and metabolic syndrome, among others.
4Fetal growth patterns (FGP) are influenced by fetal physiological and pathological characteristics, maternal factors, and the ethnic background or of the parents. All these variables contribute to significant variability in FG assessment, resulting in challenges in distinguishing between normal and pathological growth patterns.
1In current clinical practice, logarithmic regression equations are utilized for estimating fetal weight, which is incorporated into a formula
.5However, its accuracy is not absolute, as the sensitivity and specificity of this method vary significantly across different formulas.
1,5,6Hadlock’s formula, introduced in 1985, is the most widely used formula globally for calculating estimated fetal weight (EFW). It incorporates parameters such as biparietal diameter (BPD), head circumference (HC), abdominal circumference (AC), and femoral length (FL), designed for application in a North American pregnant population with a 95% confidence interval and a 10% margin of error.
7Multiple charts for fetal and neonatal growth are utilized in clinical and research settings. This practice stems from the notion that each population should develop its standards, alongside discrepancies in the definitions of small for gestational age and intrauterine growth restriction.
8For instance, in Latin America, studies have shown statistically significant differences in biometric data compared to Hadlock’s standard, prompting the development of new formulas and charts tailored to local populations.
9 However, due to the lack of local studies to determine the normal FGP in Latin American populations, the Hadlock´s charts are often used.
Efforts towards standardization and homogenization of fetal growth curves led to the establishment of the International Fetal and Newborn Growth Consortium for the 21
st Century (INTERGROWTH-21
st) project in 2009to create international fetal growth standards
.8,10 In this study, fetal growth, and birth weight of the neonates whose mothers had the best environmental, social, and medical conditions during pregnancy were measured and they observed that when these conditions are ideal, fetal growth is extremely similar among the different regions. Additionally, through this project, a new formula to calculate EFW was created that allows to follow-up fetal growth closely.
10Similarly, in Colombia, initiatives like the Colombian Research group in Fetal Growth (CRGFG) have been established to develop fetal anthropometric tables and growth curves specific to the Colombian population.
11-13 Furthermore, in 2013, Briceño conducted a study in the city of Cali measuring a total of 792 fetuses whose data showed statistically significant differences with the date of Hadlock’s charts.
14The purpose of this study was to describe the EFW using the Hadlock’s formula and the other biometric parameters in a sample of pregnant women of our population and to compare these measurements with the curves of INTERGROWTH-21
st and the curves of Lagos, to determine whether there are differences.
MethodsThis was a retrospective cross-sectional study of the data from the ultrasound reports of pregnant women from Bogotá with a singleton pregnancy and confirmed gestational age (by crown-rump length) between 16 and 39 weeks who attended the Ecodiagnóstico El Bosque Maternal-Fetal Medicine Unit between February 2015 and November 2018. Data from twin pregnancies, fetuses with major malformations, incomplete information and cases in which it was not possible to calculate gestational age by CRL at 11-13+6 weeks of gestation was excluded.
The following parameters were evaluated by ultrasound: biparietal diameter (BPD), head circumference (HC), abdominal circumference (AC), femoral length (FL), and the EFW calculated by Hadlock’s formula. Measurements were made by a specialist certified in Maternal-Fetal Medicine following the recommendations of the guidelines of The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) and guidelines of The Fetal Medicine Foundation and using the GE Voluson
TM E6 ultrasound device.
15,16For the BPD, an axial section of the fetal head at the level of the transthalamic plane was imaged, with a 90º of insonation angle, in which the cerebral hemispheres were observed symmetrical, hyperechoic middle line (falx cerebri) was interrupted by the cavum septipellucidi, and the cerebellum was not visualized. Both callipers were placed from the outer edge to the inner edge in the widest part of the skull. For the measurement of the HC, in the same section of the BPD, the ellipse was placed around the echoes of the skull bone
15or by measuring BPD and occipital-front diameter.
16.The measurement of BPD can also be taken in the transventricular plane with the technique outer edge to outer edge in terms of the location of the callipers or in the axial plane as described by Campbell and Thoms.
17To measure the AC, a cross-section of the abdomen was taken as circularly as possible, where the umbilical vein at the level of the portal sinus and the stomach bubble could be seen, and kidneys could not be seen. If the ellipse was used, the callipers were placed on the outer surface of the skin line, if not, the AC was calculated from the measurements of anteroposterior and transverse abdominal diameters. To measure these diameters, the callipers were placed on the outer edges of the contour of the body, where the widest point of the fetal abdomen was observed, then it was calculated by the formula: AC = π (APAD + TAD) / 2 = 1.57 (APAD + TAD).
18To measure the length of the femur, the view was made where the two ends of the ossified metaphysis were observed with angle of insonation of 30º. The callipers were placed at the ends of the ossified diaphysis without including the distal femoral epiphysis if it was visible, and the measurement excluded artefacts that can falsely extend the femur length.
15 In advanced gestational ages, measurement was made from the greater trochanter of the femur to the lateral condyle.
19A descriptive analysis of each ultrasonographic parameter (BPD, HC, AC and FL) was made. Atypical data were identified and reviewed. The meanand standard deviation were calculated to include the data in a graph with a representation of the 3
rd, 50
th and 97
th centiles of the INTERGROWTH-21
st project and the 10
th, 50
th, and 90
th centiles of Lagos. The distribution of each parameter in each gestational week was evaluated by the Shapiro-Wilks test and D’Agostino’s K
2 test and then these parameters were compared with the reference values of 50
th centile of INTERGROWTH-21
st project and of Lagos, by Student’s t-test and Wilcoxon signed-rank test. A value of
p<0.05 was considered statistically significant.
Ethical approval was obtained by
Ad-Hoc Ethics Committee of El Bosque University, Nº 12239 of April 02, 2014 (Approve in Session Number 9 of June 27, 2019)
, and Fundación Salud Bosque, Clínica El Bosque (Number 05-473-19).
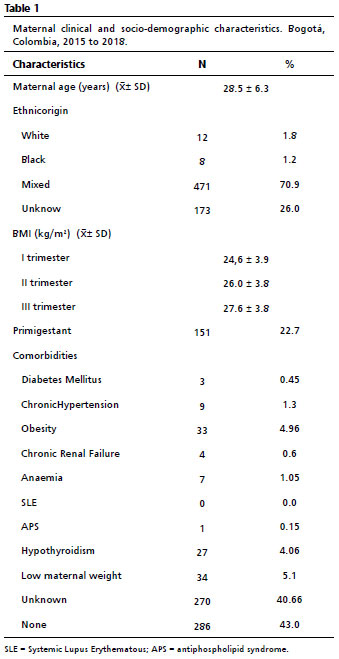
ResultsFor this study, 1150 ultrasound reports were obtained. However, 17 ultrasound reports were excluded since showed atypical data by the following specifications: typing errors in the report (n=3), stillbirth (n=1), fetal macrosomia (n=1), severe oligohydramnios (n=1), and other malformations (n=11).Therefore, in this study, data from 1133 ultrasound records were considered. The mean maternal age was 28.6 years (standard deviation 6.2), 70.9 % of patients (n=471) were from the mixed ethnic origin, the mean BMI at the first trimester was 24.6 kg/m
2 (standard deviation 3.9), and the mean BMI at the third trimester was 27.6 kg/m
2 (standard deviation 3.8). Allthe patients, 22.7% (n=151) were primigravidas. Only 4.9% of the studied pregnant women (n=33) were obese, and 5.1% (n=34) had low maternal weight. Only one patient had antiphospholipid antibody syndrome (APS), and none had systemic lupus erythematosus (SLE).
The clinical and sociodemographic characteristics of the mothers evaluated are summarized in Table 1. Additionally, distribution of the ultrasonographic measurements, the mean and standard deviation for each of the biometrics parameters and EFW per week are shown in Appendix 1.
The Figures 1 and 2 show the diagrams of box and whisker plots of the EFW and the data of each of the biometric parameters per week of the fetuses in this study.
The graphs of the estimated fetal weight of our data compared with the 3
rd, 50
th and 97
th centiles of the INTERGROWTH-21
st project and with the 10
th, 50
th and 90
th centiles of Lagos, are shown in Figure 3; and the distribution of each of the biometric parameters compared with the 3
rd, 50
th and 97
th centiles of the INTERGROWTH-21
st project and with the 10
th, 50
th and 90
th centiles of Lagos, are shown in Figure 4. The 3
rd and 97
th centiles of the INTERGROWTH-21
st project and the centiles 10
th and 90
th of Lagos, are represented by black lines and dots and the 50
th centiles of the INTERGROWTH-21
st and Lagos, are represented by the gray lines and dots. The means ± SDs of the biparietal diameter, head circumference, abdominal circumference, femur length, and estimated fetal weight measurements at 16 and 39 weeks were 34.7 ± 1.5 and 92.2 ± 4.4 mm, 122.2 ± 6.6 and 318.0 ± 17.0 mm, 107.2 ± 6.8 and 329.3 ± 34.6 mm, 20.6 ± 2.8 and 73.5 ± 3.3 mm, and 257.8 ± 20.9 and 3,115 ± 663.7g, respectively. The mean measurements of each biometric parameter from our study demonstrated significant differences compared to the 50
th percentile measurements from both the INTERGROWTH-21
st project (
p<0.05) and Lagos (
p<0.05) data across various gestational ages. Specifically, the length of the femur was longer in most cases when compared with the INTERGROWTH-21
st data (weeks 17-25 and 27-37). The biparietal diameter and head circumference were parameters that showed the most differences when compared with Lagos data (weeks 20-24, 27-36 and weeks 20-24, 27-37 respectively). Appendix 2 to 6 shows the differences between the media EFW and each of ultrasound parameters of pregnant women from our population and the 50
th centile of the INTERGROWTH-21
st project and the 50
th centile of Lagos from 16 to 40 week.
DiscussionThis study indicates that, although the curves of the biometric parameters and the EFW of fetuses in our local population have a similar distribution to those in the INTERGROWTH-21
st project and the Latin American curves of Lagos, the biometric parameter data from our population show statistically significant differences compared to these reference charts.
It is known that the alterations of fetal weight are one of the variables that most contribute to neonatal morbidity, therefore, the identification of these alterations by a closer follow-up of fetal growth is relevant to prevent an adverse perinatal outcome.
2 However, to determine a normal growth standard through the most accurate chart for each population is not easy.
There are multiple formulas to calculate the estimated fetal weight, as Hadlock’s formulas,
7 which are used in North America; like those Campbell and Thoms,
17 Shepard
et al.
20 and Warsof
et al.21 in Great Britain; Merz’s
22 in Germany and in Latin America Lagos
et al.6 and Vaccaro’s
23 formulas are the most applied. Likewise, there are also multiple population-based charts to assess fetal growth among the different ethnic groups.
6,24In Latin America, one of the largest studies was published by Araujo
et al.
25 on the Brazilian population. However, Hadlock is one of the most used formulas in the Western Hemisphere, with a margin of error of around 8.9% compared with the neonatal weight.
8INTERGROWTH-21
st project tried to obtain international standards charts for fetal measurement, while Lagos’ fetal biometrics marks standards to Chilean population. In our study, all the biometrics parameters showed a progressive increase pattern until week 35 and most of the data were between the 3
rd and 97
th centiles of INTERGROWTH-21
st project and between the 10
th and 90
th of Lagos. In these two studies, there was a slight decrease of the fetal biometric parameters at the end of the pregnancy, which results in a discrete flattening of the curve, like our study; except for the abdominal circumference whose curve continues to rise. Furthermore, the media abdominal circumference showed statistically significant differences with 50
th centile of the INTERGROWTH-21
st at advanced gestational ages and it is important to consider that these differences could affect the calculation of gestational age and the EFW, depending on the reference’s charts used.
26In the same way, it can be observed that there were significant differences between the media of FL of fetuses from our population compared with INTERGROWTH-21
st project, but not with the Lagos study, being longer the femur of our fetuses. Hammami
et al.,
27showed that the models providing the most accurate prediction of birth weight are those that include the measurements of BPD, HC, AC and FL and the most accurate model was provided by the formula of Hadlock, published in 1985. The differences of FL measurement could be relevant to the calculation of EFW in our study, although, there are some authors that considerer that FL is not determinant for the calculation of EFW, others believe that it improves its accuracy.
28,29Furthermore, it is important to consider the customized charts. In this sense, Odibo
et al.,
30 in 2018, performed a study comparing the curves of the INTERGROWTH-21
st project with a customized charts for predicting fetuses at risk of developing low birth weight and adverse perinatal outcomes, and they found that both had low sensitivity for predicting low birth weight (24.5
vs 38.8% respectively) and poor performance at predicting short-term adverse perinatal outcomes.
30 This premise could support the importance of local population charts over the customized and international growth curves.
Although the curves of the biometric parameters and the EFW of the fetuses in our population showed a similar distribution to those in the INTERGROWTH-21
st project and the Latin American curves of Lagos, the biometric parameters datafrom our population show statistically significant differences compared to these reference charts. It is important to consider these differences when evaluating fetal growth in each population.
The main contribution of this study is to define that customized or local charts are more useful for monitoringfetal growth and detecting abnormalities early. The authors consider that one of the limitations of this study is the small amount of data for some weeks, especially towards the end of pregnancy, and the limited sample of pregnant women from Bogotá, which reduces the study’s power.
With the results of this study, evaluation of fetal growth with international charts may represent a clinical issue as there was statistically significant differences of biometric parameters such as AC and FL when those were evaluated. We considered that more studies are needed to determine optimal reference charts for local populations and include studies that evaluate the correlation of the estimated fetal weight calculated by ultrasonography with the birth weight.
References1. García R, Benavides Serralde JA, Figueras Retuerta F. Can we customize fetal growth standards? Rev Colomb ObstetGinecol. 2012 Mar; 63 (Supp. l1):3-5.
2. Unterscheider J, O’Donoghue K, Daly S, Geary MP, Kennelly MM, McAuliffe FM,
et al. Fetal growth restriction and the risk of perinatal mortality-case studies from the multicentre PORTO study. BMC Pregnancy Childbirth. 2014 Feb; 14:63.
3. Chin EM, Gorny N, Logan M, Hoon AH. Cerebral palsy and the placenta: A review of the maternal-placental-fetal origins of cerebral palsy. Exp Neurol. 2022 Jun; 352: 114021.
4. Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr. 2000 May;71(5 Suppl.): 1344S-52S.
5. Souka AP, Papastefanou I, Michalitsi V, Pilalis A, Kassanos D. Specific formulas improve the estimation of fetal weight by ultrasound scan. J Matern Fetal Neonatal Med. 2014 May; 27 (7): 737-42.
6. Lagos R, Espinoza GR, Orellana JJ. New formula for estimation of fetal weight by ultrasonographic examination. Rev Hosp Mater Infant Ramón Sardá. 2002; 21 (1): 11-6.
7. Hadlock FP, Harrist RB, Carpenter RJ, Deter RL, Parl SK. Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology 1984 Feb; 150 (2): 535-40.
8. Stirnemann J, Villar J, Salomon LJ, Ohuma E, Ruyan P, Altman DG,
et al. International estimated fetal weight standards of the INTERGROWTH‐21
st Project. Ultrasound Obstet Gynecol. 2017 Apr; 49 (4): 478-86.
9. Lagos R, Espinoza GR, Echeverría P. Regional chart of normal fetal growth. Rev Hosp Mater Infant Ramón Sardá. 2002; 21 (1): 3-10.
10. Hirst JE, Papageorghiou AT. INTERGROWTH‐21
st: a new paradigm for fetal growth in the 21
st century. Obstet Gynaecol. 2016 Apr; 18 (2): 137-41.
11. Molina-Giraldo S, Romero N, Franco A. Reference values of biometry in fetal long bones between weeks 18 and 39 of gestation in the Colombian population. Rev Colomb Obstet Ginecol. 2015 Mar; 63 (Suppl. 1): 11-6.
12. Echeverry-Ciro CJ, Molina-Giraldo S, Benavides-Serralde JA. Reference values of fetal abdominal circumference between weeks 18 and 41 of gestation in the Colombian population. Rev Colomb Obstet Ginecol. 2012 Mar; 63 (Suppl. 1): 16-9.
13. Bello-Munoz Juan Carlos, Alvarado-Llano Juan José, Molina-Giraldo Saulo. Reference values of estimated fetal weight in Colombian population. Rev Colomb Obstet Ginecol. 2012 Mar ;63 (Suppl. 1): 19-21.
14. Briceño F, Restrepo H, Paredes R, Cifuentes R. Fetal size charts for a population from Cali, Colombia: sonographic measurements of biparietal diameter, head circumference, abdominal circumference, and femur length. J Ultrasound Med. 2013 Jul; 32 (7): 1215-25.
15. Salomon LJ, Alfirevic Z, Berghella V, Bilardo CM, Chalouhi GE, Da Silva Costa F,
et al. ISUOG Practice Guidelines (updated): performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol. 2022 Jun; 59 (6): 840-56.
16. Gianluigi P, Nicolaides K, Ximenes R, Jeanty P. The 18-23 weeks scan. 73
rd ed. London: Fetal Med Found; 2002. [
Internet]. [access in 2023 Oct 4]. Available from:
http://www.fetalmedicinemexico.com/the-18-23-weeks-scan-1.php17. Campbell S, Thoms A. Ultrasound Measurement of the Fetal Head to Abdomen Circumference Ratio in the Assessment of Growth Retardation. Br J ObstetGynaecol. 1977 Mar;84(3):165-74.
18. Campbell S, Wilkin D. Ultrasonic measurement of fetal abdomen circumference in the estimation of fetal weight. Br J ObstetGynaecol. 1975 Sep; 82 (9): 689-97.
19. Jeanty P, Kirkpatrick C, Dramaix-Wilmet M, Struyven J. Ultrasonic evaluation of fetal limb growth. Radiology. 1981 Jul; 140 (1): 165-8.
20. Shepard MJ, Richards VA, Berkowitz RL, Warsof SL, Hobbins JC. An evaluation of two equations for predicting fetal weight by ultrasound. Am J Obstet Gynecol. 1982 Jan;142(1):47-54.
21. Warsof SL, Gohari P, Berkowitz RL, Hobbins JC. The estimation of fetal weight by computer-assisted analysis. Am J ObstetGynecol 1977 Aug; 128 (8): 881-92.
22. Merz E, Lieser H, Schicketanz KH, Häle J. [Intrauterine fetal weight assessment using ultrasound. A comparison of several weight assessment methods and development of a new formula for the determination of fetal weight]. Ultraschall Med. 1988 Feb; 9 (1): 15-24.
23. Vaccaro H. Fetal Growth. Rev Chil Obstet Gynecol. 1991; 56:353-8.
24. Raman S, Teoh T, Nagaraj S. Growth patterns of the humeral and femur length in a multiethnic population. Int J Gynaecol Obstet. 1996 Aug;54(2):143-7.
25. Araujo Júnior E, Martins Santana EF, Martins WP, Ruano R, Pires CR, Zanforlin Filho SM. Reference charts of fetal biometric parameters in 31,476 Brazilian singleton pregnancies.J Ultrasound Med. 2014 Jul;33(7):1185-91.
26. Lagos SR, Espinoza GR, Orellana JJ. New table for estimation of fetal weight by ultrasonographic examination. Rev Chil Ultrason. 2002; 5 (1): 14-9.
27. Hammami A, Mazer Zumaeta A, Syngelaki A, Akolekar R, Nicolaides KN. Ultrasonographic estimation of fetal weight: development of new model and assessment of performance of previous models. Ultrasound Obstet Gynecol. 2018 Jul; 52 (1): 35-43.
28. Weiner CP, Sabbagha RE, Vaisrub N,
et al. Ultrasonic fetal weight prediction: role of head circumference and femur length. Obstet Gynecol. 1985 Jun; 65 (6): 812-7.
29. Woo JS, Wan CW, Cho KM. Computer-assisted evaluation of ultrasonic fetal weight prediction using multiple regression equations with and without the fetal femur length. J Ultrasound Med. 1985 Feb; 4 (2): 65-7.
Authors’ contributionVallejo Bastidas GM: conception and design of the work, analysis and interpretation of the results, writing and critical review of the manuscript. Calvo MU and Romero XC: conception and design of the work, collection/obtaining of data, analysis and interpretation of the results, and writing and critical review of the manuscript. De la Hoz-Valle J: processing, analysis and interpretation of the results, methodological and statistical advice, and critical review of the manuscript. The authors approved the final version of the article and declare that there are no conflicts of interest.
Received on November 24, 2023
Final version presented on July19, 2024
Approved on February 15, 2024
Associated Editor: Alex Sandro Souza
 ; Montserrat Uriel Calvo 2
; Montserrat Uriel Calvo 2 ; José De la Hoz-Valle 3
; José De la Hoz-Valle 3 ; Ximena Carolina Romero 4
; Ximena Carolina Romero 4