ABSTRACT
OBJECTIVES: to evaluate the effect of oropharyngeal colostrum immunotherapy in reducing cases of late-onset neonatal sepsis in preterm infants with very low birth weight.
METHODS: this is an intervention study, with a comparative analysis between the incidence of late-onset neonatal sepsis in the treatment group (in use of oropharyngeal colostrum immunotherapy) and the historical control group (newborns monitored in the same intensive care unit prior to the implementation of the oropharyngeal colostrum immunotherapy protocol). 81 premature babies born between 2016 and 2022 participated in the study, separated according to whether or not they received oropharyngeal colostrum immunotherapy. The intervention consisted of eight daily applications of 0.2 mL of the mother's own raw colostrum to the newborns oral mucosa during the first seven days of life. Relative Risk and Absolute Risk Reduction and Number needed to Treat were calculated.
RESULTS: a protective effect of oropharyngeal colostrum immunotherapy against late neonatal sepsis was noted. Relative Risk: 0.43; CI95% = 0.21-0.91; Absolute Risk Reduction: 0.26; CI95%= 6.51 - 45.92 and Number Needed to Treat: 4 (2.17-15.34).
CONCLUSION: administration of oropharyngeal colostrum proved to be a promising measure in protecting preterm newborns with VLBW against late-onset sepsis.
Keywords:
Premature newborn, Colostrum, Neonatal sepsis, Immunotherapy, Intervention studies
RESUMO
OBJETIVOS: avaliar o efeito da Imunoterapia Orofaríngea de Colostro na redução dos casos de sepse neonatal tardia, em recém-nascidos prematuros com muito baixo peso.
MÉTODOS: trata-se de um estudo de intervenção, com análise comparativa entre a incidência de sepse neonatal tardia do grupo tratamento (em uso da Imunoterapia Orofaríngea de Colostro) e grupo controle histórico (recém-nascidos acompanhados na mesma unidade de terapia intensiva neonatal, anteriormente à implementação do protocolo de Imunoterapia Orofaríngea de Colostro). Participaram do estudo 81 prematuros nascidos entre 2016 e 2022, separados de acordo com o recebimento ou não da Imunoterapia Orofaríngea de Colostro. A intervenção consistiu em oito aplicações diárias de 0,2 mL de colostro cru da própria mãe na mucosa oral dos participantes, durante os sete primeiros dias de vida. Foi feito cálculo de Risco Relativo e Redução Absoluto de Risco e do Número Necessário para Tratar.
RESULTADOS: notou-se efeito protetor da IOC contra a sepse neonatal tardia. Risco relativo: 0,43; IC95%=0,21-0,91; Redução absoluta de risco: 0,26; IC95%=6,51-45,92 e Número necessário para tratar: 4 (2,17-15,34).
CONCLUSÃO: a administração orofaríngea de colostro se mostrou uma medida promissora na proteção de RNPT com MBP contra sepse tardia.
Palavras-chave:
Recém-nascido prematuro, Colostro, Sepse neonatal, Imunoterapia, Estudos de intervenção
IntroductionPreterm newborns (PTNB), those born less than 37 gestational weeks
1, are at greater risk of complications, including infectious ones, such as neonatal sepsis.
2 Prematurity can also result in very low birth weight, birth weight below 1500 grams, a condition that increases the newborn’s vulnerability.
3Late-onset neonatal sepsis is a clinical condition resulting from an infection developed after 72 hours of life, which can be of bacterial, viral or fungal etiology, affecting body fluids such as blood, urine or cerebrospinal fluid, with frequent systemic manifestations, resulting from repercussions in organs and tissues and hemodynamic changes.
4 The immune immaturity of PTNB is involved in the sepsis physiopathology.
The supply of breast milk promotes benefits for the neonate’s immune system, helping to reduce the incidence of infection and neonatal mortality by maintaining barrier functions, protecting the gastrointestinal epithelium and inhibiting the colonization of pathogenic bacteria.
5,6However, for many PTNB weighing less than 1,500 grams at birth, it is not possible to establish enteral feeding immediately after birth, due to the clinical complications commonly presented. In addition, once started, enteral nutritional therapy is usually administered by orogastric tube, depriving the PTNB’s oropharyngeal mucosa of contact with breast milk until they are able to receive oral feedings - around 34 gestational weeks.
7Oropharyngeal colostrum immunotherapy (OCIT), with the application of maternal colostrum to the oropharyngeal mucosa prior to the start of feeding, has emerged as a strategy for immune stimulation, demonstrating a reduction in the incidence of infectious complications,
8,9 due to a reduction in the pro-inflammatory state,
10 an increase in the levels of secretory immunoglobulin A and lactoferrin
11, as well as favoring the early establishment of via parenteral nutrition.
12In view of the above, the aim of this article was to evaluate the effect of OCIT on the occurrence of cases of late-onset neonatal sepsis in PTNBs with MBP at birth.
MethodsThis is an intervention study, with a comparative analysis of the incidence of late-onset neonatal sepsis between the group exposed (treatment) and not exposed to OCIT (control). The study was conducted with PTNB with MBP at birth, born between 2016 and 2022, in a maternity at the
Sistema Único de Saúde (SUS) (Public Health System) in the State of Bahia.
The treatment group consisted of PTNB with MBP admitted to the NICU and clinically stable. The control group consisted of a historical control group, made up of PTNB with MBP admitted to the same unit between 2016 and 2018, a period prior to the implementation of the OCIT protocol in the unit, when the technique was not yet performed.
Information on clinical conditions, registered by the PTNB care team, was collected daily from medical records until hospital discharge by health professionals trained to recognize the data and fill out a specific questionnaire. The same professionals retrospectively collected data from the medical records of the historical control group.
The study included PTNBs with MBP who started receiving OCIT before 72 hours of life and who received at least 75% of the scheduled doses by the seventh day of life (minimum 42 out of 56 doses). The non-inclusion criteria were: maternal history of drugs, presence of a psychological disorder, multiparity (triplets or more) and mothers with contraindications to breastfeeding. Exclusion criteria for PTNB were: use of vasopressor drugs (> 10 mcg/Kg/min), need for immediate surgical intervention, presence of congenital syndromes or malformations and early neonatal death (in the first week of life).
The OCIT technique consisted of eight daily applications of 0.2 mL of raw colostrum (from the mother herself), on the left and right oral mucosa, during the first seven full days of life (56 applications in total). In order to start the OCIT protocol, the neonates had to be stable in the three hours prior to the application, and clinical stability was considered to be: normothermia, adequate respiratory and heart rate, normal blood pressure and oxygen saturation ≥93%. The OCIT protocol instituted at the study hospital began with the parents being referred to the Human Milk Bank (HMB) of the unit, where, after clarification, they were supported and encouraged to extract the colostrum, either manually or using a pump (Medela®).
Once extracted, the colostrum was immediately divided into 0.2 mL aliquots, kept refrigerated in sterile, disposable syringes without needles, with identification containing: the mother’s name; the date to be administered; the date, time and order number of the collection; validity (use within 12 hours) and the signature of the collector. The eight daily applications of OCIT during the seven days of the intervention were carried out by the professional nursing technicians responsible for caring for the PTNB in the NICU.
In order to define the occurrence of late-onset neonatal sepsis, the diagnosis recorded by the medical team in the PTNB’s medical records and/or the presence of a positive culture were taken into account. The PTNB care team at the unit where the study was carried out follows the following recommendations for diagnosing neonatal sepsis: at least one positive culture in body fluid after 72 hours of life, laboratory alterations such as leukocytosis (leukocytes >25000 mm3), or leukopenia (leukocytes <5000 mm3) with left shift, plateletopenia (platelets <150000 p/mm3) and elevated C-Reactive Protein (CRP) (>6 mg/L).
In addition to cases of sepsis defined by the medical diagnosis and/or positive culture, late-onset neonatal sepsis was also considered when the PTNB used antibiotics for more than five days, required a change in antibiotic regimen after five days of life and/or the introduction of antifungal drugs, associated with clinical signs of infection: thermal instability, respiratory alterations and the laboratorial alterations mentioned above. The cutoff time of five days was chosen because it has been identified in the scientific literature as the definition of clinical sepsis/suspected sepsis.
13,14 The time of antibiotic use was assessed according to the start and end date of the medication (time of life of the PTNB at the time).
The neonatologists located in the NICU usually follow the routine previously defined for premature infants, with the first starting regimen consisting of antibiotics with a lower spectrum of action (ampicillin or crystalline penicillin G and gentamicin), the second (oxacillin and amikacin) and third regimen (cefepime, vancomycin, meropenem, ceftraxiona, clindamycin, ceftadizime).
The sample calculation was carried out using the EPI INFO 7.2.5.0 software, using the following parameters: 80% power, 5% sampling error, 95% confidence interval, 1:1 ratio, 92% prevalence of neonatal sepsis and relative risk of 1.84, according to the study by Lee et al.,
11 totaling 44 participants and adding a 15% loss forecast, with a need for 52 participants, 26 in the exposed group and 26 in the control group.
The intervention took place by convenience, so that all PTNBs who met the criteria would be able to receive the OCIT and take part in the study after maternal invitation and informed consent.
In the descriptive analysis, the simple and relative frequencies of the categorical variables and covariates were obtained. The normality of the data was assessed using the Shapiro-Wilk test, which confirmed the asymmetry of the data.Medians and interquartile ranges were used as measurements of central tendency and dispersion, respectively, for the quantitative data.
Due to the aforementioned issue, the Mann-Whitney test was used to compare the continuous covariates between the groups.Pearson’s chi-square test and Fisher’s exact test were used as appropriate (number of events per cell) to assess the distribution of categorical variables between the comparison groups.
The analysis of late-onset neonatal sepsis was adjusted for maternal age, marital status, gestational hypertension, type of delivery, number of prenatal visits and birth weight.The relative risk (RR) and absolute risk reduction (ARR) and the number needed to treat (NNT) were calculated.
This study was submitted and approved by the Ethics Committee of the State
Universidade Estadual de Feira de Santana (UEFS), under protocol number 2.930.252. The study is registered with the Brazilian Clinical Trial Registry (ReBEC) under registration RBR-2cyp7c and UTN U1111-1222-0598.
ResultsFigure 1 describes the flowchart for recruiting the PTNBsparticipants taking part in the study.
Table 1 describes the maternal sociodemographic characteristics and birth conditions of the NB participating in the study. The majority of the women were over 18 years old, self-reported as black, had completed high school, but were mostly in unpaid work and marital status was single, divorced or widowed; they were primiparous and had not undergone full prenatal care, evolving to natural premature birth with a rupture of amniotic membranes of less than 24 hours, with no registration of maternal infection in the peripartum period (urinary, chorioamnionitis, group B streptococcus and other peripartum infections). As for the NB, there were more premature babies with gestational age ≥28 weeks at birth and weighing more than 1,000 grams. In addition, 81% of the NB in the sample had an early infection and/or were at risk of infection.
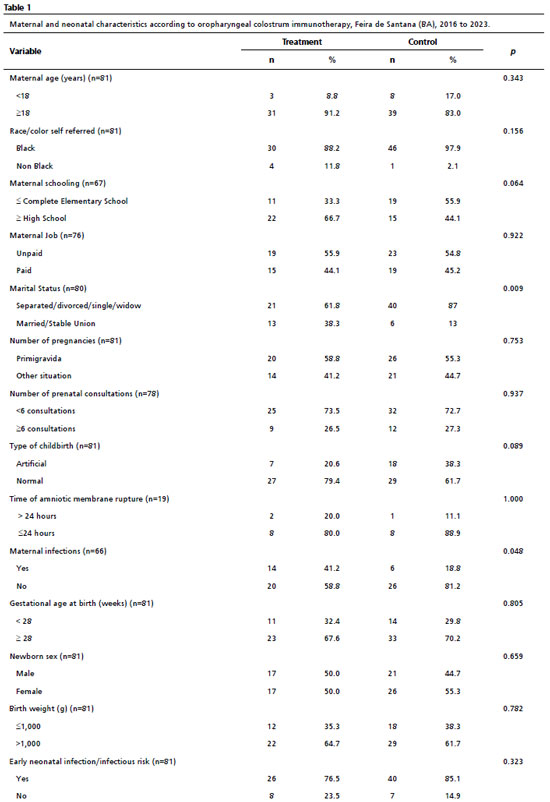
Table 2 shows the NBs’ clinical and hospitalization conditions. With regard to the use of antimicrobials by PTNBs, in the treatment and control groups the median time for starting antibiotic use was the first day of life. In the treatment group, it was observed that premature infants received OCIT remained on a central venous catheter for less time (
p-value = 0.040), but were in a longer time on a peripherally inserted central catheter (
p-value = 0.008) and were older when parenteral nutrition was suspended (
p-value = 0.029).
The need for therapeutic antifungals (after 72 hours of life) in the sample was 18.5%, corresponding to 15 uses, four in the treatment group and 11 in the control group (
p=0.250).
Changes of antibiotic regimen occurred in approximately 69% of cases (55 NB), of which six NB started using antibiotics from the second regimen, with a subsequent change to a higher spectrum regimen. Of these 55 antibiotic regimen changes, 22 occurred in the treatment group and 33 in the control group (
p=0.502). Regarding the changes made, ten NB used the second regimen after 72 hours of life, while 53 NB used the third regimen after the same period.
There are changes in N in Tables 1 and 2 for some outcomes due to the absence of information in the medical records of some participants in the study.
The assessment of late-onset sepsis is shown in Table 3. The relative risk of 0.43 (CI95%=0.21-0.91) indicated that OCIT acted as a protective factor against the risk of developing late-onset neonatal sepsis, with a 26% reduction in cases as a result of its use (RAR=0.26; CI95%=6.51-45.92). The number needed to treat (NNT) was four (CI95%=2.17-15.34). Of the 29 cases of late-onset sepsis in the sample, 15 were confirmed by medical diagnosis and/or blood culture and 14 were considered late-onset sepsis based on the clinical sepsis/suspected sepsis criteria mentioned in the methodology.
DiscussionThe incidence of late-onset neonatal sepsis was lower in the treatment group when compared to the control group, confirming the assumption that OCIT is a protective factor against this pathology. Similar results were observed in the study by Ouyang
et al.
14 carried out in China, in which the same diagnostic criteria for late-onset sepsis were applied and no type of intervention was used in the control group (routine care), where it was found that the number of cases of late-onset sepsis was lower in the treatment group than in the control group. Similarly, the study by Sudeep
et al.15 published in 2022 in India, using an OCIT protocol very similar to the one applied in this study, also showed a reduction in late-onset sepsis in the treatment group.
In line with the results found, a recent systematic review with meta-analysis, published in 2023 by Yan
et al.
16 showed that OCIT is effective in reducing the incidence of late-onset neonatal sepsis, a fact also supported by other systematic reviews published in 2022
9 and 2020,
17 although the review produced by Slouha et al.
18 did not observe the same findings.
Similarly, Leonardo
et al.
19 found a higher incidence of sepsis in the control group than in the group treated with pasteurized maternal colostrum in a study carried out in an Italian hospital. Unlike the aforementioned study, the present study used raw maternal colostrum in the OCIT, with extraction within the first 72 hours after chilbirth.
The preference for applying raw colostrum to the oral mucosa lies in the fact that the heat treatment of human milk, used in pasteurization, causes a reduction in bioactive components, such as immunoglobulins, lactoferrin, lysozyme and cytokines.
20 In turn, performing OCIT with raw colostrum preserves the activity of the immunocomponents that perform bactericidal/bacteriostatic functions, a fact that may have contributed to the reduction in the incidence of late-onset sepsis in the treatment group.
It is pertinent to comment on the clinical conditions of the preterm newborns included in the sample. It was noted that the time taken to suspend parenteral nutrition and PICC was longer in the treatment group than in the control group. The use of the intravenous route is a fundamental item in the care of PTNBs, especially for the administration of antibiotics and parenteral nutrition. Although these data may suggest a worse outcome in this group, it can be hypothesized that the treatment group had a longer time using these devices, as they had a greater chance of being treated (longer ICU stay) and of surviving. A study by Martins
et al.
21 was carried out with the same population and the same study site showed that the use of oropharyngeal colostrum reduced the risk of death in the intervention group.
Still on the subject of the use of PICCs, when compared to central venous catheters, this procedure presents a lower risk of complications, such as systemic and insertion complications.
22 Because of this, there has been a significant increase in the use of this type of device in recent years, as well as an increase in the number of professionals trained to insert them. Based on this change in the care landscape, it is possible to conjecture that the greater use of PICCs in the treatment group, compared to the control group, was also due to the difference in the time of data collection for the two groups (control from 2015 to 2018 and treatment from 2019 to 2022).
To explain the older age of the treatment group at the time parenteral nutrition was discontinued, one could use the same reasoning that the increased chance of survival and lower neonatal mortality associated with OCIT
21 implied a longer time spent using hospital resources, such as parenteral nutrition.
It is relevant to note that the type and time of intravenous device used, as well as the time of parenteral nutrition, can be explained by possible changes in the care model at the time of the control group and the treatment group.
A trend was observed, without statistical significance, suggesting that the control group required a greater change in the antibiotic regimen and also a greater need to use antifungals after 72 hours of life, which indicates a tendency for the infection to worsen in this group and corroborates the findings of a greater number of sepses for these NB. In line with the cases of sepsis in the population, the use of higher spectrum antibiotics, such as vancomin, meropenem and cefepime, are typically used in the treatment of late-onset neonatal sepsis.
23 The need for antifungals can also be explained by the vulnerability of PTNBs to fungal infections.
24No reduction in antibiotic use time was observed with the use of OCIT. The systematic review by Nasuf et al.
25 published in 2018 also showed no impact of OCIT on the use and days of antibiotic use. It is likely that the use of empirical antibiotic therapy from birth in both groups masked the results.
This therapeutic approach, started in the first few days of life to manage early sepsis and/or infectious risk, is in line with the literature which shows the widespread use of antibiotics as prophylaxis for early sepsis.
23,26 Also from this perspective, the use of empirical antibiotic therapy for more than five days increases the risk of late-onset neonatal sepsis, especially in NICUs where there is widespread use of broad-spectrum antibiotics and low use of breast milk.
26,27In regard to maternal characteristics, it should be noted that the mothers in the treatment group had a higher prevalence of infections, a characteristic that is known to increase the risk of infection in newborns,
26 who will consequently require greater vigilance in the treatment of infections.This fact also contributes to reinforcing the protective role of OCIT, since it would be expected that the group of NBs born to mothers with a higher prevalence of infections would have a higher risk of developing neonatal sepsis.
Still on maternal characteristics, the number of women with marital statuses such as separated, divorced, single or widowed was significantly more prevalent in the control group than in the group treated with OCIT. Certainly, the absence of a formal partner or the lack of recognition of paternity increases the chances of premature birth,
28,29 as well as its negative consequences.
A limitation of the current study is the need to use a historical control group rather than a placebo control group at the same time as the treatment group; however, this was required by the ethics committee to which the study was submitted. It is important to consider the use of the mother’s own raw colostrum as immunological therapy for a population that is usually subjected to prolonged use of antibiotics, long hospital stays, prolonged use of parenteral nutrition, late initiation of parenteral nutrition with breast milk, factors that alter the intestinal microbiota and increase the risk of neonatal sepsis due to pathogenic colonization.
27,30The oropharyngeal administration of colostrum proved promising as a protective factor against late-onset sepsis, with an absolute reduction in the risk of this pathology in 26% of the newborns in the treatment group and a major clinical impact, as the treatment of four very low birth weight premature newborns was enough to prevent one event from occurring.From a public health point of view, this result strengthens the case for OCIT in the context of neonatal care, as a measure to prevent late-onset neonatal sepsis, a pathology that has a high neonatal morbidity and mortality rate, especially for premature infants.
References1. World Healthy Organization (WHO). Preterm Birth. [Internet] [acess in 2023 Mai 10]. Available from:
https://www.who.int/news-room/fact-sheets/detail/preterm-birth2. Collins A, Weitkamp JH,Wynn JL. Why are preterm newborns at increased risk of infection? Arch Dis Child Fetal Neonatal Ed. 2018 Jul; 103 (4): F391-4.
3. Fleiss N, Tarun S, Polin RA. Infection prevention for extremely low birth weight infants in the NICU. Seminars. Fetal Neonatal Med. 2022; 27 (3): 101345.
4. Shane AL, Sánchez PJ, Stoll, BJ. Neonatal sepsis. Lancet. 2017; 390 (10104): 1770-1780.
5. Lewis ED, Richard C, Larsen BM, Field CJ. The Importance of Human Milk for Immunity in Preterm Infants. Clin Perinatol. 2017; 44 (1): 23-47.
6. Li D-F, Shi C-X, Zhao L, Shi F-Z, Jiang M-L, Kang W-Q. Prevention of neonatal ventilator-associated pneumonia through oral care with the combined use of colostrum and sodium bicarbonate. Eur Rev Med Pharmacol Sci. 2021; 25 (5): 2361-6.
7. Rodriguez NA, Vento M, Claud EC, Wang CE, Caplan MS. Oropharyngeal administration of mother’s colostrum, health outcomes of premature infants: study protocol for a randomized controlled trial. Trials. 2015; 16 (1): 453.
8. Cai M, Lin L, Peng Y, Chen L, Lin Y. Effect of Breast Milk Oral Care on Mechanically Ventilated Preterm Infants: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Pediatr. 2022; 10: 899193.
9. Huo M, Liu C, Mei H, Zhang Y, Liu C, Song D,
et al. Intervention Effect of Oropharyngeal Administration of Colostrum in Preterm Infants: A Meta-Analysis. Front Pediatr. 2022; 10: 895375.
10. Martín-Álvarez E, Diaz-Castro J, Peña-Caballero M, Serrano-López L, Moreno-Fernández J, Sánchez-Martínez B,
et al. Oropharyngeal Colostrum Positively Modulates the Inflammatory Response in Preterm Neonates. Nutrients. 2020; 12 (2): 413.
11. Lee J, Kim HS, Jung YH, Choi KY, Shin SH, Kim EK,
et al. Oropharyngeal colostrum administration in extremely premature infants: an RCT. Pediatrics. 2015; 135 (2): e357-366.
12. Ramos MDSX, Martins CDC, Souza ES, Vieira GO, Gomes-Filho IS, Figueiredo ACMG,
et al. Oropharyngeal colostrum immunotherapy and nutrition in preterm newborns: meta-analysis. Rev Saúde Pública. 2021; 55: 59.
13. Glass K, Greecher C, Doheny K. Oropharyngeal Administration of Colostrum Increases Salivary Secretory IgA Levels in Very Low-Birth-Weight Infants. Amer J Perinatol. 2017; 34 (14): 1389–95.
14. OuYang X, Yang CY, Xiu WL, Hu YH, Mei SS, Lin Q. Oropharyngeal administration of colostrum for preventing necrotizing enterocolitis and late-onset sepsis in preterm infants with gestational age ≤ 32 weeks: a pilot single-center randomized controlled trial. Int Breastfeed J. 2021; 16 (1): 59.
15. Sudeep KC, Kumar J, Ray S, Dutta S, Aggarwal R, Kumar P. Oral Application of Colostrum and Mother’s Own Milk in Preterm Infants—A Randomized, Controlled Trial. Indian J Pediatr. 2022; 89 (6): 579-86.
16. Fu ZY, Huang C, Lei L, Chen LC, Wei LJ, Zhou J,
et al. The effect of oropharyngeal colostrum administration on the clinical outcomes of premature infants: A meta-analysis. Int J Nurs Stud. 2023; 144: 104527.
17. Tao J, Mao J, Yang J, Su Y. Effects of oropharyngeal administration of colostrum on the incidence of necrotizing enterocolitis, late-onset sepsis, and death in preterm infants: a meta-analysis of RCTs. Eur J Clin Nutr. 2020; 74 (8): 1122-31.
18. Slouha E, Anderson ZS, Mansa N, Kalloo AE, Vasavi RG. Colostrum and Preterm Babies: A Systematic Review. Cureus. 2023; 15 (7): e42021.
19. Leonardo SWM, Ilao MAL, Juico MM. Efficacy of Oropharyngeal Administration of Pasteurized Colostrum in Very Low Birthweight Newborns in Reducing Late Onset Sepsis at a Tertiary Government Hospital in Manila City: A Randomized Control Trial. Acta Med Philipp. 2022; 56 (16).
20. Wesolowska A, Sinkiewicz-Darol E, Barbarska O, Bernatowicz-Lojko U, Borszewska-Kornacka MK, van Goudoever JB. Innovative Techniques of Processing Human Milk to Preserve Key Components. Nutrients. 2019; 11 (5): 1169.
21. Martins CC, Ramos MDSX, Amaral MVC, Costa JSP, Cerqueira ES, Vieira TDO,
et al. Colostrum oropharyngeal immunotherapy for very low birth weight preterm infants: protocol of an intervention study. BMC Pediatr. 2020; 20 (1): 371.
22. Bahoush G, Salajegheh P, Anari AM, Eshghi A, Aski BH. A review of peripherally inserted central catheters and various types of vascular access in very small children and pediatric patients and their potential complications. J Med Life. 2021 Jun; 14 (3): 298-309.
23. Puopolo SK. Infecções Bacterianas e Fúngicas. In: Cloherty JP, Eichenwald EC, Stark AR. Manual de neonatologia. 7
th ed. Rio de Janeiro: Guanabara Koogan; 2015. p. 490-515.
24. Weimer KED, Smith PB, Puia-Dumitrescu M, Aleem S. Invasive fungal infections in neonates: a review. Pediatr Res. 2021; 91 (2): 404-12.
25. Nasuf AWA, Ojha S, Dorling J. Oropharyngeal colostrum in preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2018; 9 (9): CD011921.
26. Procianoy RS, Silveira RC. The challenges of neonatal sepsis management. J Pediatr (Rio J). 2020; 96 (S1): 80-6.
27. Dong Y, Speer CP. Late-onset neonatal sepsis: recent developments. Arch Dis Child Fetal Neonatal Ed. 2015; 100 (3): F257–F263.
28. Thoma ME, Drew LB, Hirai AH, Kim TY, Fenelon A, Shenassa ED. Black–White Disparities in Preterm Birth: Geographic, Social, and Health Determinants. Am J Prev Med 2019; 57 (5): 675-86.
29. Lessa MSA, Nascimento ER, Coelho EAC, Soares IJ, Rodrigues QP, Santos CAST,
et al. Pré-natal da mulher brasileira: desigualdades raciais e suas implicações para o cuidado. Ciênc Saúde Colet. 2022; 27 (10): 3881-90.
30. Madan JC, Salari RC, Saxena D, Davidson L, O’Toole GA, Moore JH,
et al. Gut microbial colonisation in premature neonates predicts neonatal sepsis. Arch Dis Child Fetal Neonatal Ed. 2012; 97 (6): F456–F462.
Authors’ contributionSantos GC: data collection and analysis, writing of the manuscript. Vieira TO: methodological design, data analysis, revision of the manuscript. Martins CC: methodological design, data collection and analysis, revision of the manuscript. Costa MGR: data collection, revision of the manuscript. Vieira GO: project administration, acquisition of funding, methodological design, revision of the manuscript. All the authors have approved the final version of the article and declare no conflicts of interest.
Received on April 28, 2024
Final version presented on August 24, 2024
Approved on August 29, 2024
Associated Editor: Gabriela Buccini
 ; Tatiana de Oliveira Vieira 2
; Tatiana de Oliveira Vieira 2 ; Camilla da Cruz Martins 3
; Camilla da Cruz Martins 3 ; Matheus Gomes Reis Costa 4
; Matheus Gomes Reis Costa 4 ; Graciete Oliveira Vieira 5
; Graciete Oliveira Vieira 5